Cuff Pressure Algometry
Quantitative, objective pain assessment that enables a mechanism-based quantification of pain.


Chronic musculoskeletal pain is linked with sensitisation, initially in the peripheral structures and often later sensitisation of central mechanisms [1]. The degree of sensitisation can be estimated using standardised quantitative sensory testing (QST)[2]. The initial protocols for QST mainly focused on peripheral sensitisation in neuropathic pain, but modern QST protocols for musculoskeletal pain focus on three main aspects: widespread hyperalgesia, facilitation of pain in the central nervous system and the central nervous system’s ability to inhibit pain.
Cuff algometry provides standardised QST protocols focusing on bilateral pressure pain thresholds, temporal summation of pain (TSP) and conditioning pain modulation (CPM). Cuff algometry is an evidence-based research tool which has shown good to excellent within and between-day reliability in healthy subjects and patients with chronic musculoskeletal pain (ICC > 0.7) [3], [4], and it is used widely to study pain mechanisms in healthy subjects and chronic pain patients. Customisable protocols can be tailored to fit your specific protocol if the standardised protocols are not sufficient.
Widespread hyperalgesia facilitated TSP and impaired CPM have been studied in pain patients ranging from adolescents with patellofemoral pain [5] to patients with knee osteoarthritis [6] to patients with fibromyalgia [7] using cuff algometry.
Studies focusing on phenotyping of pain patients have shown that groups of patients with facilitated TSP and impaired CPM assessed by cuff algometry have more widespread pain in a general chronic pain patient population [8]. Further, the same methodology has been used to screen patients with knee osteoarthritis before total knee replacement. Patients with facilitated TSP and impaired CPM seem to be at a higher risk of developing chronic postoperative pain one year after surgery [6]. Further, widespread preoperative hyperalgesia measured by cuff algometry has been associated with the development of chronic postoperative pain following total knee replacement [6], and preoperative TSP has been associated with postoperative pain following total hip replacement [9].
With Cuff Pressure Algometry, a subject is presented with a pressure stimulus while they rate the sensation with either a rating scale or indicate that threshold(s) has been reached with a button. The type of pressure stimulation determines which mechanisms are studied. Below are examples of test procedures that are possible with Cuff Pressure Algometry.
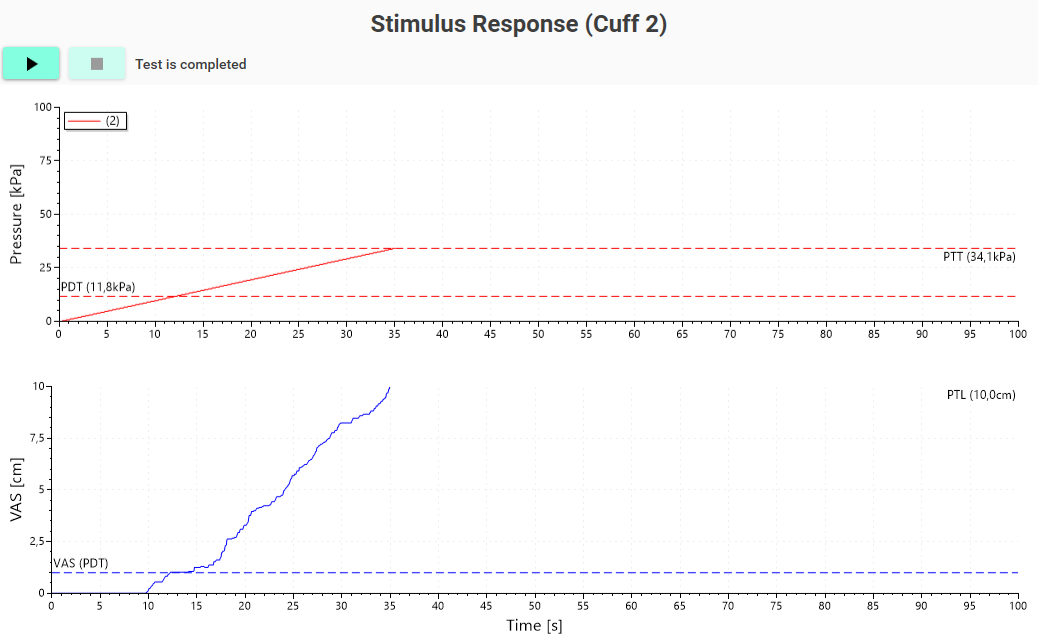
Pain detection and Pain tolerance thresholds are determined with stimulus-response curves, a procedure in which the pressure is linearly increased. At the same time, the subject rates the pain on a rating scale or stops the stimulation with a button; the system can automatically calculate pain detection thresholds (PDT), pain tolerance threshold (PTT), and the pressure required to elicit a given VAS score from the subject.
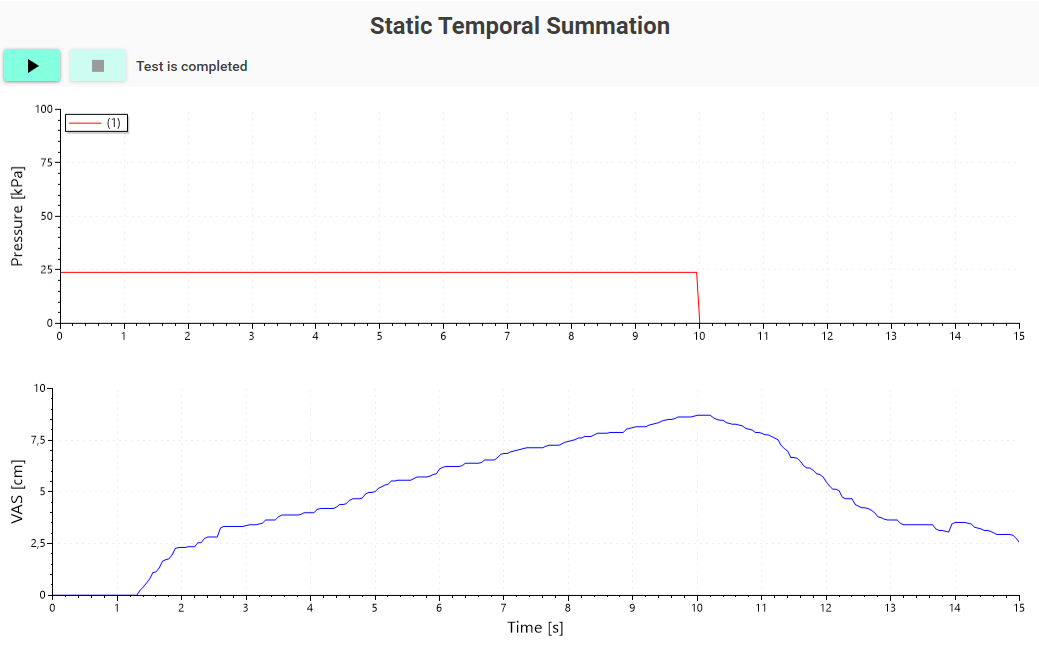
A response-dependent protocol in which pressure stimuli of the same intensity ( typically the individual PTT) are applied repetitively while the subjects rate the pain intensity. Temporal summation is then determined based on the subjects´ pain response to the stimuli. Standard and evidence-based protocols are available.

Spatial summation can be performed both with response-dependent and stimulus-dependent protocols. With spatial summation, the same protocol ( e.g. PDT/PTT) is performed repeatedly with two cuffs with a different surface stimulation area ( or two equally sized cuffs placed next to each other).
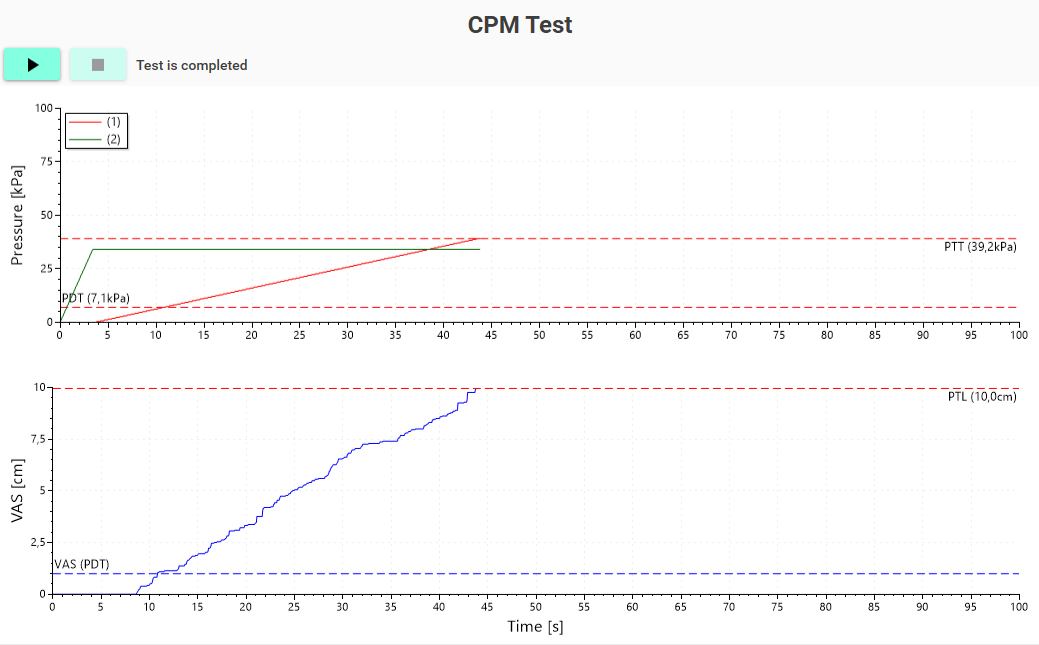
Conditioned pain modulation is performed using two cuffs at two different extremities. One cuff is used for conditioning and inflated to a constant painful pressure stimulation, while the other cuff is used for stimulus-response assessment of the pain detection and tolerance thresholds.
Arbitrary pressure stimulations can be defined by linear piecewise segments. With this capability, the experimenter can develop and use their own procedures for Cuff Pressure Algometry.

The LabBench CPAR+ Due and Uno are devices for cuff pressure algometry. These devices offer computer-controlled pressure stimulations, enabling protocols that are impossible with conventional handheld pressure algometry.
As the pressure stimulations are computer controlled, it is possible to perform protocols that require precise temporal control over the applied pressure. Possible protocols with the LabBench® CPAR+ devices are the determination of cuff pressure pain detection and tolerance thresholds, temporal summation, spatial summation, and conditioned pain modulation.
The CPAR+ devices can synchronise external 3rd party equipment with a digital trigger output and be triggered by a digital input. This enables the CPAR+ devices to be used in experiments where biophysical signals such as EEG are recorded.
The devices have a form factor of A4 paper (30 x 21 x 10cm) and weigh 3kg. This enables them to be easily transported between experimental sites. They require an external air supply, which can be supplied either through readily available and low-cost standard air compressors, compressed air bottles (i.e. diving bottles) or compressed air systems.
Nocitech is part of LabBench®, a platform for neuroscience studies within cuff pressure algometry, nerve excitability testing, and quantitative sensory testing. Click Learn More to learn more about LabBench and how to get started with Cuff Pressure Algometry.
Quantitative, objective pain assessment that enables a mechanism-based quantification of pain.
Inventors' Way ApS
Niels Jernes Vej 10
DK9220 Aalborg, Denmark
CVR.NR. 37596108
Copyright© Inventors' Way ApS